|
Down load your own copy of our publication:DEWATERING 2nd Edtn
Erosion Control Guidelines: Section IV Dewatering
|
A Guide to Understanding Dewatering Second Edition, May, 2011
By Gordon Peabody, Safe Harbor Environmental Services
Edited by Audra Mickunas, Safe Harbor Environmental Services
This material is considered Public Domain
Synopsis: When water volumes are pumped from a source to a discharge area, impacts may occur to each of those habitats. We need to review the chemical, biological and physical signatures of habitats at both the source and discharge areas. We also need to calculate discharge volumes as a function of time, to assess potential impacts. For example, thermal mass differential is important when considering lethality indices of aquatic and marine species. pH is also a key water quality parameter, influencing a wide array of hydrochemical and biochemical functions.
A. Dewatering Definitions
1. Short or long term removal of a water volume encountered when the scope of a project requires temporary access below sea level or within the groundwater lens.
2. Removal of water is determined to be necessary, in order to facilitate safe installation of structural components or utilities.
3. Dewatering may be “same source” (FW to FW or well; salt water to salt water).
4. Dewatering may be “conflicting sources” (FW to salt water; FW to dry land)
5. Discharge volume is pumped to dry land, wetland or open water.
6. Characteristics of Cape Cod Aquifer:
a. Rainfall percolates through sandy soils into the fresh water lens.
b. Horizontal migration moves ground water towards shorelines.
c. This fresh water lens may elevate and subside with tidal cycles.
f. Lens elevations are reflected in wetland and pond levels.
g. Cape water bodies are all groundwater fed, not discharge fed.
B. Scope
1. Small scale: boats, basements, foundations.
2. Medium (meso) scale : wharf construction, roadway.
3. Large (macro) scale : river crossing.
4. These guidelines will discuss meso scale dewatering.
C. Duration
1. Short term: 24-72 hours or periodic.
2. Long term: Extended, 24/7 for up to 100 days.
D. Volume
1. Low volume: (5K GPH) Gasoline or diesel powered pumps.
2. Medium volume: (10-100 KGPH) Diesel or electric pumps.
3. High volume: (+1 MGD) Electric generators provide power.
E. Techniques
1. Collection:
a. Open hose: direct removal of ground water from excavation.
b. Well extraction: well points are inserted adjacent to the area.
2. Discharge:
a. Direct: open hose
b. Indirect: diffuser; filter bag; filter sock; stabilization tank.
c. Closed (limited) site: pond; swale; bog, field swale; well.
d. Open (unconfined) site: intertidal; open water; open field areas.
F. Impacts
1. Removal site impacts:
a. Depression of the water table may impact adjacent tree roots.
b. Depression of water table may impact adjacent vernal pools.
c. Removal of water can mobilize contaminants at source.
2. Closed site discharge impacts:
a. Physical impacts
i. Existing water could be displaced.
ii. High volume could alter circulation in wetlands.
iii. High velocity could erode subsurface sediments.
iv. Introduced sediment and silt may alter phonic zone.
v. Sediment and silt could smother vegetation.
vi. direct discharge of ground water is habitat anomaly.
b. Chemical impacts: Source pH could vary from discharge pH
i. Altered pH could create chemical precipitation.
ii. Altered pH could weaken macro, micro invertebrates
(Ion rebalancing requires excess bio energy).
iii. Altered pH could reduce micro rhizome function .
iv. Lower pH could impact phytoplankton exoskeletons.
c. Biological impacts
i. Altered temperatures impact metabolism rates
ii. Altered temperatures impact dissolved oxygen
iii. Discharge could introduce nutrients
iv. Discharge flow could free sediment nutrients
v. Temperature stratification may be interrupted
3. Open intertidal discharge site:
a. Physical impacts
i. High volume could create chronic erosion patterns
ii. High velocity could create abnormal erosion or
generate sediment transport
iii. Sediment and silt transport may alter photic zones
iv. Deposition may impact benthic invertebrates
v. Direct discharge of ground water is habitat anomaly
b. Chemical impacts:
i. Groundwater pH would be lower than seawater.
ii. Lower pH could create chemical precipitation .
iii. Lower pH could weaken macro and micro invertebrates
(ion rebalancing requires bioenergy).
iv. Lower pH could alter available nutrients.
v. PH below 7 reduces phytoplankton and larval
Invertebrate’s absorption of exoskeletons.
vi. Discharge may contain different dissolved gasses.
c. Biological impacts:
i. Altered temperatures impact metabolic rates.
ii. Thermal shock from discharge can be lethal to organisms iii. Altered temperatures impact dissolved oxygen (temperature and DO have an inverse relationship).
iv. Discharge could introduce additional nutrients.
v. Silt and sediment loading can reduce light levels.
vi. High volume, long term freshwater flow would alter
localized benthic population and diversity.
vii. Discharge may be a vector for bacteria.
G. Mitigations
1. Velocity, volume and duration:
a. Reduce pumping.
b. Phase pumping.
c. Reduce discharge intensity with manifold system.
d. Reduce discharge intensity with diffuser system.
e. Reduce point source impacts with multiple lines.
f. Discharge using spray heads.
2. Suspended particles:
a. Eliminate particles at source with filters.
b. Use well points with filter screens.
c. Use filter bag at discharge point.
d. Discharge into straw lined surface swale.
e. Discharge into excavated swale.
f. Use settling tank.
3. Temperature:
a. Open surface waters may reflect air temperatures.
b. Ground water will reflect Earth temperature.
c. Differentials may require above grade holding basin for adjustment.
d. Spray heads can expose ground water to air temperature.
e. Reverse differentials may require diffused discharge.
4. Chemical:
a. Cape ground water pH is approximately 5.5 -6.5.
b. FW Wetlands may also have low, slightly acidic pH.
c. Sea water pH is usually between 7.9-8.2.
d. Holding tank would allow adjustment of pH.
e. Filter bag would allow adjustment of pH .
f. Swimming pool chemicals can adjust pH before discharge.
H. Illustrations
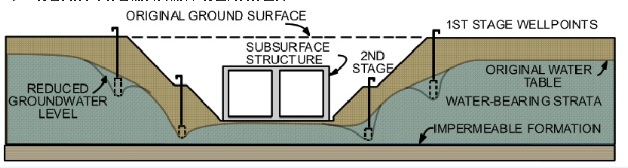
1. Meso scale, using wells for dewatering. No discharge shown.
2. Dewatering bags can modify sediment and temperature signature
3. Filter bags can be used for meso scale dewatering.
4. holding tanks can be used to modify pH, sediment and temperature signature.
5. Filter screens on well points can resuce sediment transfer.
6. Manifolds and diffusers reduce discharge site impacts.
|
|
|
|
|
|
|
|
|
|
|
|
|
|